PA Department of Health Guidance Following COVID-19 Vaccination
The Pennsylvania Department of Health (DOH) provides guidance in 2020-PAHAN-541 for responding to signs and symptoms following COVID-19 vaccination in health-care personnel (HCP). Strategies are needed for health-care facilities to appropriately evaluate and manage post-vaccination signs and symptoms among health-care personnel (HCP) in order to minimize staffing disruptions and the transmission of infectious diseases, including COVID-19.
Key points in the advisory include:
- Signs and symptoms – such as fever, fatigue, headache, chills, myalgia, and arthralgia – can typically occur following COVID-19 vaccination. They usually occur within the first three days of vaccination.
- Presence of signs and symptoms consistent with SARS-CoV-2 infection that are not typical for post-vaccination signs and symptoms (i.e. cough, shortness of breath, rhinorrhea, sore throat, loss of taste or smell) should not be attributed to the COVID-19 vaccine.
- Positive viral (nucleic acid or antigen) tests for SARS-CoV-2, if performed, should not be attributed to the COVID-19 vaccine since vaccination does not influence the results of these tests.
A figure is provided to outline the steps health-care facilities should take in response to HCP who develop symptoms within the three days following vaccination for COVID-19. If you have questions about this guidance, please contact DOH at 1-877-PA-HEALTH (1- 877-724-3258) or your local health department.
Positive Approaches Journal Available – The Data Issue
Advocates Demand Virtual Access to Allegheny County Criminal Courts, “It’s Not Difficult”
The COVID Vaccine Is Here — What Does that Mean for PA Schools?
Updates for ICF/IDD Providers

Message from Kevin Dressler:
COVID-19 Vaccine
The Food and Drug Administration (FDA) has approved the Pfizer vaccine, and the Moderna vaccine may be approved by the end of the week. The federal government has been distributing vaccines to each of the states, and the Pennsylvania Department of Health (DOH) has released the Pennsylvania COVID-19 Interim Vaccination Plan for distribution and administration of the vaccine. The Interim Plan places the intermediate care facilities (ICFs) in Phase 1A of the vaccine administration. We do not have any information on the actual dates for the vaccine administration at this time.
Post Vaccination
DOH has released HAN 541, which offers guidance for health-care workers on post-vaccination signs and symptoms. It offers some clarifying information on symptoms that may be expected as a result of receiving the vaccine and symptoms unrelated to the vaccine, which may indicate that a person has COVID-19.
Reduction of Quarantine Time
In my previous email, I indicated that some guidance was forthcoming that could reduce the time staff would need to quarantine. The guidance did come out in HAN 538, but it does not apply to health-care settings, and it is for the community at large.
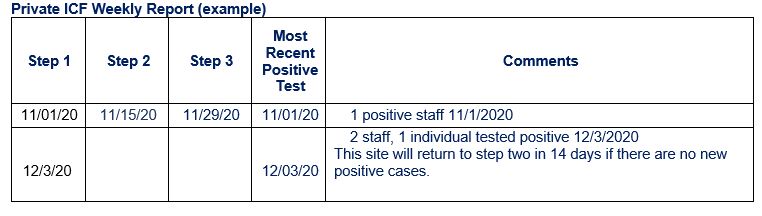
Private ICF Weekly Report
This is a reminder that each week, the Private ICF Weekly Report needs to be submitted to your respective regional lead for each ICF location. There has been some confusion on how the dates for each reopening step and most recent positive test should be entered into the report. The “Most Recent Positive Test” applies to staff and people served by the location in which the report is submitted. I have included an example below showing how the form should be filled out with the appropriate comments. If you have any further questions regarding the weekly report, please reach out to your regional ICF lead.
As the Gap Between Students and Teachers of Color Widens in PA, Black Families Demand Change
RCPA Member Goodwill Keystone Area Receives Historic $10 Million Donation
CMS Issues CY 2021 Update for Durable Medical Equipment, Prosthetics, Orthotics, and Supplies Fee Schedule
The Centers for Medicare and Medicaid Services (CMS) issued Medicare Learning Network (MLN) Matters article MM12063 entitled “Calendar Year (CY) 2021 Update for Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Fee Schedule”. It becomes effective on January 1, 2021. This article includes material on the data files, update factors, and other information related to the update of the fee schedule. The DMEPOS fee schedule is updated on an annual basis. For CY 2021, an update factor of 0.2 percent is applied to certain DMEPOS fee schedule amounts. Additional details specific to supplies are provided in the article.