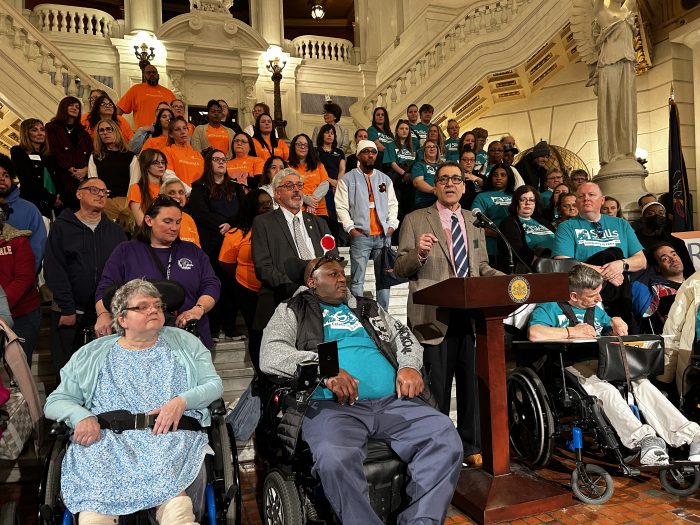Approval Antics: Navigating the Drug Maze of IDD & BH — Tarrytown Expocare Pharmacy Webinar on April 10
RCPA Member BHARP Announces Justin Wolford as New COO
Federal COVID-Related SUD Grants to Continue While Courts Consider Request for Temporary Restraining Order

The Pennsylvania Department of Drug and Alcohol Programs (DDAP) notified single county authorities (SCA) that it will continue to distribute federal COVID-related grant funding to SCAs while the US District Court considers a 23-state lawsuit seeking an emergency temporary restraining order against US Health and Human Services Secretary Robert F. Kennedy Jr. for abruptly terminating COVID-grant funds that were appropriated for use for states until September 30, 2025. Those grant funds include a supplemental to the Substance Use Disorder Block Grant. The lawsuit was filed on April 1.
Though not confirmed, media reports suggest the termination of grants could cost the Pennsylvania Department of Health $301 million, along with an additional $28 million or more hit against DDAP.
DDAP had been using these grant funds to expand testing and provide resources for COVID; support providers and help meet local needs during the pandemic; and expand the substance use disorder prevention, intervention, treatment, and recovery support services continuum, including various evidence-based services and supports for individuals, families, and communities.
OMHSAS Peer Support Services Stakeholders Quarterly Meeting
The Pennsylvania Department of Human Services Office of Mental Health and Substance Abuse Services (OMHSAS) will be holding a quarterly public meeting on Tuesday, April 8, 2025, 10:00 am to 11:00 am, for anyone interested in discussing the topic of peer support services (PSS) while working in the mental health field. In particular, OMHSAS will be providing updates on staffing and the release of frequently asked questions.
The Microsoft TEAMS link for this meeting can be found below.
Please send any discussion topics, questions, or agenda items via email by April 7. The email account will be monitored, and the sender will receive a reply only if more information is needed.
If you or your colleagues would like to be added to an OMHSAS Listserv to receive the quarterly invitations, please visit this website and then click on the “join or leave the list” link.
Meeting Date/Time: Agenda Topics Due By:
Tuesday, 1/14/25, 10:00 am December 30, 2024
Tuesday, 4/8/25, 10:00 am April 7, 2025
Tuesday, 7/8/25, 10:00 am June 24, 2025
Tuesday, 10/14/25,10:00 am September 30, 2025
OMHSAS is open to ideas and suggestions on maximizing the effectiveness of these meetings.
These quarterly meetings are not a replacement for the Mental Health Planning Council quarterly meetings; those will continue in addition to these newly established meetings.
Questions pertaining to these meetings should be submitted via email.
TEAMS MEETING INFORMATION:
Microsoft Teams Need help?
Join the meeting now
Meeting ID: 223 156 162 141
Passcode: QkkY9M
Please contact RCPA Policy Associate Emma Sharp with any questions or concerns.
PA Among 23 Plaintiffs Suing US HHS Secretary RFK Jr. Over Termination of COVID Grants
Governor Shapiro and Pennsylvania are listed along with 22 other plaintiffs in a lawsuit filed in US District Court in Rhode Island, requesting an emergency temporary restraining order against US Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. for abruptly terminating COVID-grant funds, including a supplemental to the Substance Use Disorder Block Grant, that were appropriated for use for states until September 30, 2025.
Though not confirmed, media reports suggest the termination of grants could cost the Pennsylvania Department of Health $301 million, along with an additional $28 million or more hit against the Pennsylvania Department of Drug and Alcohol Programs (DDAP).
DDAP had been using these grant funds to expand testing and provide resources for COVID; support providers and help meet local needs during the pandemic; and expand the substance use disorder prevention, intervention, treatment, and recovery support services continuum, including various evidence-based services and supports for individuals, families, and communities.
DDAP is examining its options to maintain the full array of services offered by single county authorities and their providers to ensure Pennsylvanians continue to receive the lifesaving supports they need.
The factual allegations and legal background in the lawsuit state that during the COVID-19 pandemic, Congress appropriated substantial funds to strengthen public health programs that were not tied to the duration of the public health emergency. HHS and Congress continued to make these public health funds available after the end of the pandemic.
On Monday, March 24, with no advance notice, HHS abruptly terminated $11 billion in grants and cooperation agreements funded by appropriations from COVID-related laws. States were notified through letters from the Substance Abuse and Mental Health Administration (SAMHSA). The letters indicated the grants were issued for a limited purpose: to ameliorate the effects of the pandemic. The end of the pandemic provides cause to terminate COVID-related grants. Now that the pandemic is over, the grants are no longer necessary.
The lawsuit goes on to state the terminations have caused and will continue to cause irreparable harm and asks the court to vacate and set aside the termination of the funding and any other further actions taken by US HHS to implement or enforce them, among other requests.
Positive Approaches Journal Vol. 13, Issue 4 Released
The Intersection of Mental and Physical Health Impacting our Communities: Part 1
The Office of Developmental Programs (ODP) and the Office of Mental Health and Substance Abuse Services (OMHSAS) are pleased to announce the latest edition of the Positive Approaches Journal.
This issue of the Positive Approaches Journal looks at topics related to challenges around maintaining and supporting both physical and mental health, as well as information on resources. This issue looks at the shortage of both primary health and mental health care professionals, and the five primary health conditions that can significantly elevate the risk of severe illness and death in individuals with ID/A. Other topics include: supporting the nutritional needs of individuals with autism, utilizing a public health approach to address wellness for individuals who have encountered trauma, and supporting individuals and their families who have survived suicide.
This issue of Positive Approaches Journal is in digital form, available for viewing online or for downloading. To print a copy of the PDF, online journal, or a specific article, you will find these options within your left navigation bar on any Positive Approaches Journal page. A new window will open with your selected document. In your browser, you may click the Print button in the top left corner of the page, or by using the Print capability within your browser.
Please submit feedback regarding your experience with the Positive Approaches Journal on MyODP by selecting the feedback image on MyODP within your left navigation bar on any Positive Approaches Journal page.
The Positive Approaches Journal is published quarterly. For additional information, please contact ODP Training’s inbox.
Federal Medicaid Update From National Council
Sponsor, Exhibit, and Advertise at the 2025 RCPA Conference “Strive to Thrive”
RCPA is excited to share the growing list of sponsors and exhibitors who are committed to supporting our 2025 Conference Strive to Thrive! This event, which will be held September 9 – 12 at the Hershey Lodge, is a highlight for the PA health and human services fields. We would like to thank the organizations who have committed their support already; you can view them below and on our Conference website!
There are still many opportunities available for sponsorship and exhibit booths, and we encourage your organization to view this year’s Sponsors, Exhibitors, and Advertisers Brochure, which features detailed lists of all the ways your organization can thrive at our conference. These include networking opportunities in Connections Hall and new sponsorship items. Please be aware booth self-selection will also be available for exhibitors and exhibiting sponsors. In order to be considered for self-selection, a completed contract with payment must be submitted.
Sign Up Now!
Sponsors, exhibitors, and advertisers who wish to be listed on the website, the mobile app, and in the conference program must submit all materials by August 20. The association looks forward to welcoming you at the conference! Space and opportunities are reserved on a first-come, first-served basis, and no reservation is considered complete without payment. Please contact Carol Ferenz, Conference Coordinator, with any questions.
View our sponsors and exhibitors at our Conference website!
RCPA, PA Lawmakers Spotlight Urgent Need for Fair Funding, Workforce Investment in Human Services
HARRISBURG, PA — A bipartisan group of Pennsylvania lawmakers joined members of the Rehabilitation and Community Providers Association (RCPA), the Commonwealth’s largest health and human services trade association, at a press conference at the state Capitol in support of vital disability and human service programs. These programs serve millions of Pennsylvanians annually and play a transformative role in their lives.
At the March 26 press conference, RCPA and lawmakers pushed for continued funding and improved payment models, including Medicaid capitation, as well as decreasing administrative burden in the safety net system, as part of any final 2025/26 budget adopted by the General Assembly. These initiatives will help improve services and make the system work better for everyone. They also highlighted the need to invest in the workforce, ensuring strong support for licensed clinicians, direct support professionals, counselors, case managers and support/service coordinators, and peers.
Richard S. Edley, PhD, President and CEO of RCPA, spoke on behalf of members and those who rely on health and human services. Fady Sahhar, MBA, PhD, RCPA Director of Physical Disabilities & Aging, also communicated the need for Medicaid preservation and continued funding.
| Richard S. Edley, PhD, President & CEO | Fady Sahhar, MBA, PhD, Director of PD&A |
 |
 |
RCPA members also raised their voices to stress the importance of not only maintaining but improving the systems in place. Speakers included Melva Fair, an RCPA Board Member and CEO of Community Living and Support Services (CLASS), and Annie Smith, Director of Early Intervention at RCPA member Strawberry Fields. Also in attendance were RCPA Board Members Susan Coyle of Chartiers Center and Gretchen Kelly of PLEA.
| Melva Fair | Annie Smith | Susan Coyle and Gretchen Kelly |
 |
 |
 |
Representatives from both sides of the aisle in the House and Senate spoke in agreement with RCPA’s message, voicing continued support for vital services in Pennsylvania.
| Representative Doyle Heffley | Representative Joseph Hohenstein | Senator Tim Kearney |
 |
 |
 |
Last but not least, RCPA thanks everyone who attended this year’s Capitol Day. Your support and presence made this year one of our most successful press conferences to date!
 |
 |
 |
 |