On February 3, 2026, Governor Josh Shapiro delivered his fourth budget address to the Pennsylvania General Assembly. He began the address by commending the significant growth of the Commonwealth over the last three years, highlighting improvements in quality education, agriculture, energy, and becoming the only state in the Northeast with a growing economy.
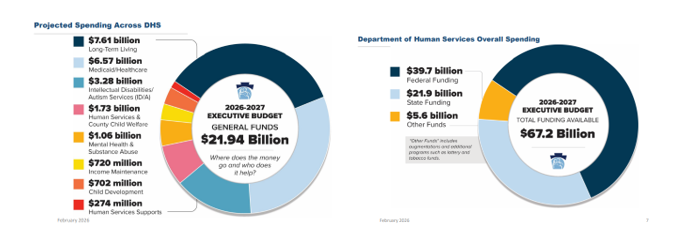
Shapiro’s 2026/27 proposed budget calls for $53.3 billion in state General Funds expenditures, with Medicaid (32%) and Education (36%) being the largest spend categories. Other expenditures include:
- Human Services and other DHS programs (9%);
- Corrections and Parole (6%);
- Higher Education (4%);
- Debt Service (3%); and
- 10% remaining for all other categories.
The 2026/27 budget also includes supplemental appropriations of $390.3 million to increase the FY 2025/26 General Fund spend to $51.5 billion. The majority of this increase is attributed towards DHS programs and Education through the end of FY 2025/26, with a $374.5 million increase for human services related to increased projections of utilization, caseloads, and enrollments anticipated through June 2026.
Similar to last year’s address, Shapiro called for the legalization and taxation of adult use cannabis as well as taxing and regulating skill games terminals in order to support the proposed 2026/27 expenditure. The budget would also be funded by transferring $4.6 billion from the Budget Stabilization Reserve Fund (Rainy Day Fund) to the General Fund, which would leave the Rainy Day Fund balance at $3.3 billion.
2026/27 Proposed Budget Highlights:
Behavioral Health
- Behavioral Health Medicaid Capitation: The 2026/27 proposed budget includes a 15% increase to $4.4 billion in Medicaid capitation funding to the behavioral and physical HealthChoices programs. This is the amount of money from which behavioral health Medicaid managed care organizations reimburse providers for mental health and SUD treatment services. At this point, how the total capitation funding breaks out between behavioral and physical health is not known. The current fiscal year budget includes $1.95 billion in behavioral health Medicaid capitation.
- Substance Use Disorder Funding: The Department of Drug and Alcohol Programs saw a $300,000 increase in its general operations fund but was otherwise flat-funded. Most of DDAP’s state dollars are passed onto Single County Authorities to fund treatment at the county level.
- Adult Use Recreational Marijuana: The proposed budget included $730 million in anticipated revenue from legalized adult use recreational marijuana, which, if legalized by the legislature, would take effect January 1, 2027.
- Mental Health Services: The proposed budget includes a $65 million (+6.9%) increase, including $10 million to support the 988 network, $7.3 million to expand diversion and discharge programs for individuals with mental illness currently in the criminal justice system, and $5 million to maintain walk-in mental health crisis stabilization centers.
- School Mental Health: Shapiro proposed $100 million for school mental health and safety, totaling in $400 million over his term.
Intellectual and Developmental Disabilities
- Intellectual Disabilities Community Waiver: The Governor’s proposed FY 2026/27 budget includes a $76.8 million increase for the Intellectual Disabilities Community Waiver line item, largely intended to maintain existing services and enrollment rather than expand capacity. No funding is currently explicitly proposed for waiting list initiatives.
- ARPA HCBS Funding: One-Time ARPA HCBS funding is ending in 2026. A limited backfill is proposed to continue select initiatives, raising questions about the long-term sustainability of workforce and service enhancements supported with one-time federal funds.
- Minimum Wage Increase: The proposal to increase the minimum wage to $15/hour, effective January 1, 2027, would significantly impact IDD providers without a clearly identified, corresponding waiver rate adjustment in the budget.
- Emphasis on Stability: Overall, the budget proposal shows a focus on maintaining current system stability. Ongoing workforce cost pressures and rate adequacy remain key concerns for IDD service providers.
Community HealthChoices
- The initial release of the Governor’s 2026/27 budget for the Department of Human Services (DHS) includes an increase of 7.47% for Medical Assistance/Community HealthChoices. RCPA will be working with DHS to determine what this increase to the CHC-MCOs will encompass and how this could impact members under the Community HealthChoices program.
While this brief overview provides a snapshot of the Governor’s proposed budget, RCPA Policy Staff will be working with our lobbying partners, healthcare experts, and systems’ stakeholders to provide a thorough analysis of the budget to members. We have confirmed that DHS will be hosting a virtual proposed budget briefing on its portion of the State Budget funding this Friday, February 6, 2026. An invitation was distributed to RCPA members earlier this morning. The virtual briefing will provide some additional details, but the DHS “Blue Book” provides the most detail on certain appropriation lines and should be available in the coming weeks in advance of its hearings with the Senate and House Appropriations Committees.
If you have questions, please contact your respective RCPA Policy Director.













 Highlight new policy, research, and treatment initiatives, such as the use of artificial intelligence and technology in service provision;
Highlight new policy, research, and treatment initiatives, such as the use of artificial intelligence and technology in service provision; If the proposal is accepted, individuals must be prepared to present on any day of the conference. Workshops are 90 minutes in length. If the topic requires an in-depth presentation, a double session can be scheduled for a total of 180 minutes. At the time of acceptance, presenters will be asked to confirm the ability to submit workshop slides and handouts electronically two weeks prior to the conference. Individuals unable to meet this expectation may not have their materials available to participants during the conference.
If the proposal is accepted, individuals must be prepared to present on any day of the conference. Workshops are 90 minutes in length. If the topic requires an in-depth presentation, a double session can be scheduled for a total of 180 minutes. At the time of acceptance, presenters will be asked to confirm the ability to submit workshop slides and handouts electronically two weeks prior to the conference. Individuals unable to meet this expectation may not have their materials available to participants during the conference.