Updates for ICF/IDD Providers

Message from Kevin Dressler:
COVID-19 Vaccine
The Food and Drug Administration (FDA) has approved the Pfizer vaccine, and the Moderna vaccine may be approved by the end of the week. The federal government has been distributing vaccines to each of the states, and the Pennsylvania Department of Health (DOH) has released the Pennsylvania COVID-19 Interim Vaccination Plan for distribution and administration of the vaccine. The Interim Plan places the intermediate care facilities (ICFs) in Phase 1A of the vaccine administration. We do not have any information on the actual dates for the vaccine administration at this time.
Post Vaccination
DOH has released HAN 541, which offers guidance for health-care workers on post-vaccination signs and symptoms. It offers some clarifying information on symptoms that may be expected as a result of receiving the vaccine and symptoms unrelated to the vaccine, which may indicate that a person has COVID-19.
Reduction of Quarantine Time
In my previous email, I indicated that some guidance was forthcoming that could reduce the time staff would need to quarantine. The guidance did come out in HAN 538, but it does not apply to health-care settings, and it is for the community at large.
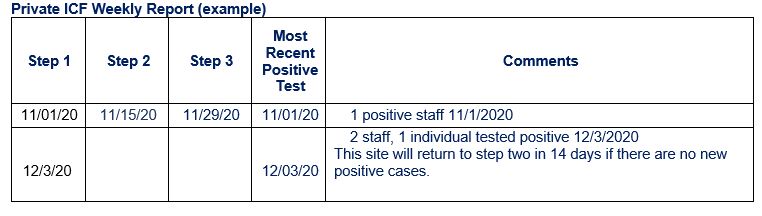
Private ICF Weekly Report
This is a reminder that each week, the Private ICF Weekly Report needs to be submitted to your respective regional lead for each ICF location. There has been some confusion on how the dates for each reopening step and most recent positive test should be entered into the report. The “Most Recent Positive Test” applies to staff and people served by the location in which the report is submitted. I have included an example below showing how the form should be filled out with the appropriate comments. If you have any further questions regarding the weekly report, please reach out to your regional ICF lead.